Nuclear physics

Nuclear physics
Lecture №1
Introduction in nuclear physics

Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions. Other forms of nuclear matterare also studied. Nuclear physics should not be confused with atomic physics, which studies the atom as a whole, including its electrons.
Discoveries in nuclear physics have led to applications in many fields. This includes nuclear power, nuclear weapons, nuclear medicineand magnetic resonance imaging, industrial and agricultural isotopes, ion implantation in materials engineering, and radiocarbon datingin geology and archaeology. Such applications are studied in the field of nuclear engineering.
Particle physics evolved out of nuclear physics and the two fields are typically taught in close association. Nuclear astrophysics, the application of nuclear physics to astrophysics, is crucial in explaining the inner workings of stars and the origin of the chemical elements.
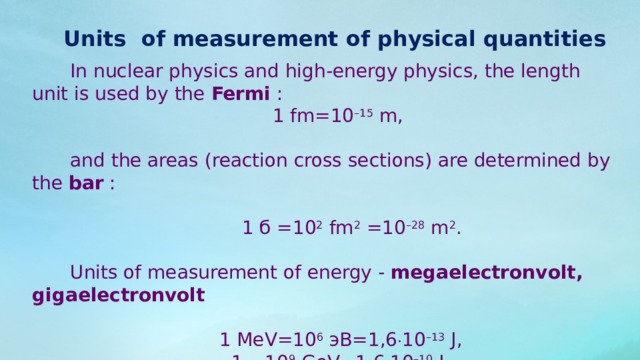
Units of measurement of physical quantities
In nuclear physics and high-energy physics, the length unit is used by the Fermi :
1 fm=10 –15 m,
and the areas (reaction cross sections) are determined by the bar :
1 б =10 2 fm 2 =10 –28 m 2 .
Units of measurement of energy - megaelectronvolt, gigaelectronvolt
1 MeV=10 6 эВ=1,6 10 –13 J,
1 =10 9 GeV=1,6 10 –10 J.

The mass of elementary particles is measured by MeV / c 2 , where c is the speed of light in a vacuum, c = 3 10 8 m / s.
Sometimes the mass is measured in MeV.
For example, the electron mass is 0.51 MeV, and the proton is 938 MeV.
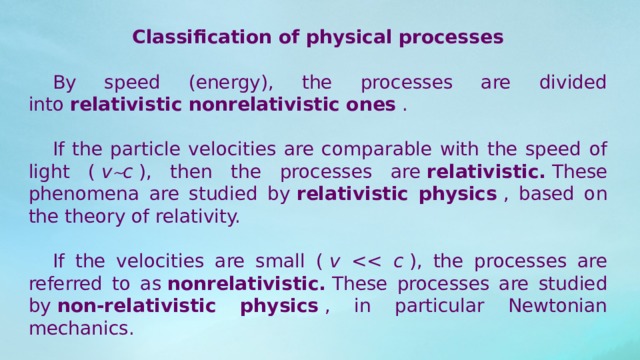
Classification of physical processes
By speed (energy), the processes are divided into relativistic nonrelativistic ones .
If the particle velocities are comparable with the speed of light ( v c ), then the processes are relativistic. These phenomena are studied by relativistic physics , based on the theory of relativity.
If the velocities are small ( v ), the processes are referred to as nonrelativistic. These processes are studied by non-relativistic physics , in particular Newtonian mechanics.
 100 million light years is valid for characteristic scales, the concept of megahir is considered. The science that studies the properties and evolution of the megaworld is called cosmology . The bodies surrounding the human body have "ordinary" dimensions and make up the macrocosm - the subject of macroscopic physics . If the inequality R microworld, which is studied by quantum physics. " width="640"
100 million light years is valid for characteristic scales, the concept of megahir is considered. The science that studies the properties and evolution of the megaworld is called cosmology . The bodies surrounding the human body have "ordinary" dimensions and make up the macrocosm - the subject of macroscopic physics . If the inequality R microworld, which is studied by quantum physics. " width="640"
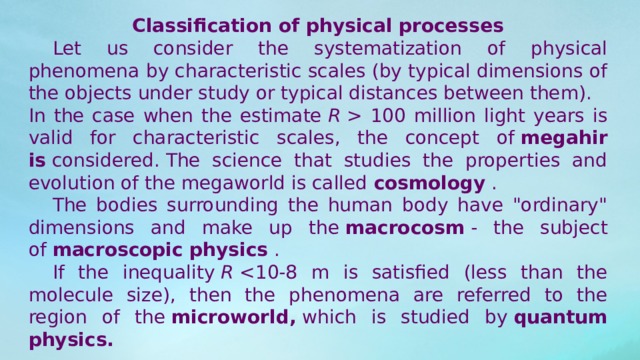
Classification of physical processes
Let us consider the systematization of physical phenomena by characteristic scales (by typical dimensions of the objects under study or typical distances between them).
In the case when the estimate R 100 million light years is valid for characteristic scales, the concept of megahir is considered. The science that studies the properties and evolution of the megaworld is called cosmology .
The bodies surrounding the human body have "ordinary" dimensions and make up the macrocosm - the subject of macroscopic physics .
If the inequality R microworld, which is studied by quantum physics.

The microcosm has a "fine structure":
10-10
R 10-15 m - objects of nuclear physics and physics of low-energy particles;
10-15
R
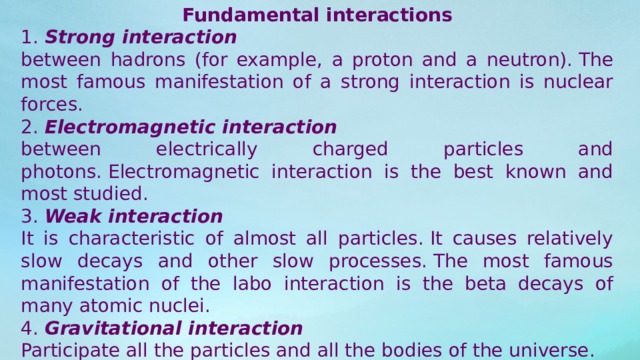
Fundamental interactions
1. Strong interaction
between hadrons (for example, a proton and a neutron). The most famous manifestation of a strong interaction is nuclear forces.
2. Electromagnetic interaction
between electrically charged particles and photons. Electromagnetic interaction is the best known and most studied.
3. Weak interaction
It is characteristic of almost all particles. It causes relatively slow decays and other slow processes. The most famous manifestation of the labo interaction is the beta decays of many atomic nuclei.
4. Gravitational interaction
Participate all the particles and all the bodies of the universe.
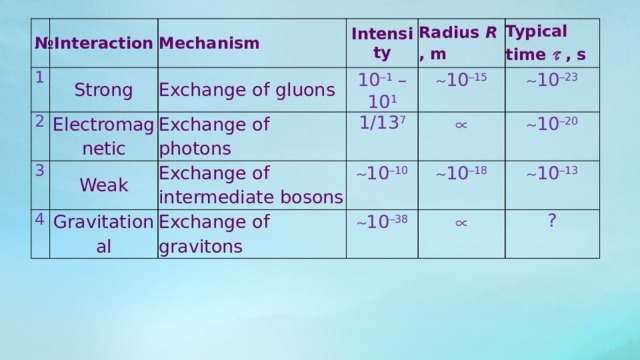
№
Interaction
1
2
Mechanism
Strong
Intensity
Electromagnetic
3
Exchange of gluons
Weak
4
Radius R , m
Exchange of photons
10 1 – 10 1
Gravitational
Typical time , s
Exchange of intermediate bosons
1/13 7
10 15
10 10
10 23
Exchange of gravitons
10 20
10 18
10 38
10 13
?

The discovery of the atomic nucleus
1816 - U. Prout hypothesized that all atoms are composed of hydrogen atoms. However, subsequent measurements of the atomic masses of the elements showed that they are not a multiple of the atomic mass of hydrogen.
1869 - D. Mendeleev built a periodic system of elements.
1886 - U. Crookes suggested that the atoms consist of a primary substance called "protil".
1901 - J.Peren published an article "Nuclear-planetary structure of the atom": electrons move around the positive nucleus, the frequencies of revolution of which coincide with the frequencies of light emitted by this atom.
1903 - JJ Thompson formulated a static model of the structure of the atom - "pudding with raisins".
1904 - H.Nagaoka by analogy with the planet Saturn came to the planetary model of the atom.
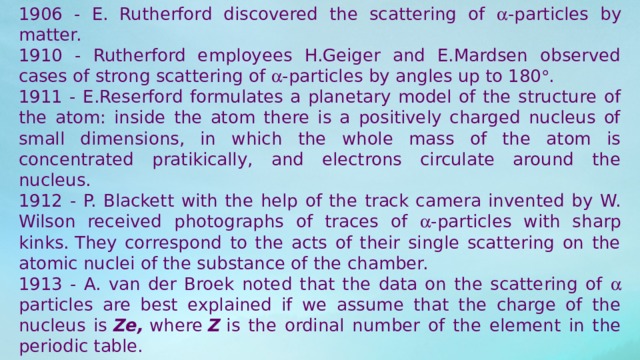
1906 - E. Rutherford discovered the scattering of -particles by matter.
1910 - Rutherford employees H.Geiger and E.Mardsen observed cases of strong scattering of -particles by angles up to 180 .
1911 - E.Reserford formulates a planetary model of the structure of the atom: inside the atom there is a positively charged nucleus of small dimensions, in which the whole mass of the atom is concentrated pratikically, and electrons circulate around the nucleus.
1912 - P. Blackett with the help of the track camera invented by W. Wilson received photographs of traces of -particles with sharp kinks. They correspond to the acts of their single scattering on the atomic nuclei of the substance of the chamber.
1913 - A. van der Broek noted that the data on the scattering of particles are best explained if we assume that the charge of the nucleus is Ze, where Z is the ordinal number of the element in the periodic table.
1914 - G. Mosley, measuring the frequencies of characteristic X-ray radiation, confirmed the hypothesis of Van der Broek.

Nuclear installations of Kazakhstan
Institute of Nuclear Physics (Almaty)
Research reactor WWR-K
The basin-type reactor on thermal neutrons was commissioned in 1967. Demineralised water is used as a coolant, retarder and reflector.
The maximum density of thermal neutrons is 1.1х1014 n / (cm2s).
The thermal power is 6 MW

Nuclear installations of Kazakhstan
Institute of Nuclear Physics (Almaty)
Critical stand
Studies are underway on a low-power (100 W) thermal neutron reactor. Here, light water and beryllium are used as a moderator and reflector.

Nuclear installations of Kazakhstan
Institute of Nuclear Physics (Almaty)
Accelerator of heavy ions UKP-2-1
Electrostatic charge-exchange accelerator was put into operation in 1987.

Nuclear installations of Kazakhstan
Institute of Nuclear Physics (Almaty)
Isochronous cyclotron U-150M
The cyclotron in the "classical mode" was commissioned in 1965.
The energy of accelerated particles
10 MeV / nucleon.
In 1972 is isochronous.
Energy: protons 7-30 MeV,
The helium-3 ions are 18-61 MeV,
The helium-4 ions are 25-50 MeV.

Nuclear installations of Kazakhstan
Institute of Nuclear Physics (Almaty)
Electronic accelerator ELV-4
It was launched in 1993. The first installation of electron-beam technologies in Kazakhstan.

Nuclear installations of Kazakhstan
Institute of Nuclear Physics (Astana)
Accelerator complex DC-60
Composition of the accelerating complex DC-60:
- Heavy ion injector-implantator;
- axial beam injection system;
- isochronous cyclotron;
- ion output system;
- 3 channels for transportation of accelerated ions;
- channel for transporting low-energy ions;
- scientific and technological installations.

Nuclear installations of Kazakhstan
National Nuclear Center (Kurchatov)
Tokamak KTM
It was put into operation in September 2010.
The purpose of the work is to obtain a breakdown of the working gas in a vacuum chamber and the formation of a plasma cord with a current of 10-30 kA.
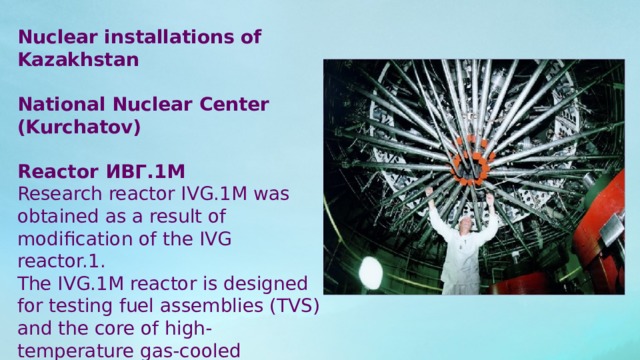
Nuclear installations of Kazakhstan
National Nuclear Center (Kurchatov)
Reactor ИВГ.1М
Research reactor IVG.1M was obtained as a result of modification of the IVG reactor.1.
The IVG.1M reactor is designed for testing fuel assemblies (TVS) and the core of high-temperature gas-cooled reactors.
.

Nuclear installations of Kazakhstan
Nuclear installations of Kazakhstan
National Nuclear Center (Kurchatov)
IGR reactor
The pulse-graphite reactor is a source of neutrons and gamma rays.

Nuclear installations of Kazakhstan
Mangystau atomic power complex (Aktau)
Reactor BN-350
The fast neutron reactor, intended for testing the materials used in nuclear reactors, was decommissioned in 1999.










 100 million light years is valid for characteristic scales, the concept of megahir is considered. The science that studies the properties and evolution of the megaworld is called cosmology . The bodies surrounding the human body have "ordinary" dimensions and make up the macrocosm - the subject of macroscopic physics . If the inequality R microworld, which is studied by quantum physics. " width="640"
100 million light years is valid for characteristic scales, the concept of megahir is considered. The science that studies the properties and evolution of the megaworld is called cosmology . The bodies surrounding the human body have "ordinary" dimensions and make up the macrocosm - the subject of macroscopic physics . If the inequality R microworld, which is studied by quantum physics. " width="640"