«Water biology. Why water is the basis of life»
Introduction
The purpose of the lesson is to get acquainted with the structure of the water molecule and its role in the life of the cell.
Water. Total value
Water is one of the most common substances on our planet. For many living organisms, water is doubly important, because it is not only part of their cells, but also a habitat (pic. 1).

Pic. 1. Water as a habitat. Part of a coral reef
In the cell in quantitative terms, water ranks first among all chemical compounds.
Water in the body is free and bound.
Free water is part of the cytoplasm of the cell, vacuoles; it fills the intercellular space, blood vessels, the space between organs - it is necessary for the transport and transfer of substances.
Bound water is part of cellular structures (proteins, membranes) and supports their structure.
Water has a number of properties that are extremely important for living organisms. The unique properties of water are determined by the structure of its molecule.
Water molecule
A water molecule consists of an oxygen atom and two hydrogen atoms.
The oxygen atom, as more electronegative than the hydrogen atoms, pulls the electron density back on itself. As a result, it shifts in its direction, and a partially positive charge appears on the hydrogen atoms, and a partially negative charge appears on the oxygen atom.
Since the atoms in the water molecule form an angle (Fig. 2), one end of the water molecule carries a positive charge, and the other end carries a negative charge. Such a molecule is called a dipole (Fig. 2), or a polar molecule.
Hydrogen bond
The partial positive charge of the hydrogen atom of one molecule interacts with the partial negative charge of the oxygen atom of another molecule. An electrostatic interaction occurs between them, and hydrogen bonds are formed. Hydrogen bonds are weak, but there are quite a lot of them in water, so the unique properties of water are determined by the presence of hydrogen bonds in water.

Given this ability of water, consider those properties of water that are important from a biological point of view.
Biologically important properties of water
Water is a universal solvent. It is an excellent solvent for polar compounds. These include ionic compounds, such as salts, in which charged particles, ions, dissociate, that is, they separate from each other in water when the substance dissolves.
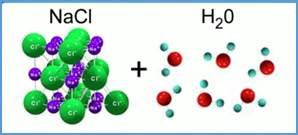

The dissolution of polar substances in the ATS. The formation of ions with a hydration shell
As well as compounds, such as sugars and simple alcohols, whose molecules contain charged groups, that is, these substances have functional groups for interacting with water.

Hydrolysis of sucrose in water
In solution, the molecules or ions of a substance begin to move faster, and the reactivity of this substance increases. All biochemical processes take place in aqueous solutions.
Polar substances "lipids" do not mix with water, and therefore can divide water solutions into separate compartments. Nonpolar parts of molecules are repelled by water, and in its presence are attracted to each other.
Nonpolar molecules interact with water in a different way-they gather in drops and form films. Such substances are called hydrophobic.


Oil is a substance that does not dissolve in water – it forms films on the surface of water (left) or collects in drops (right)
Such hydrophobic interactions play an important role in ensuring the stability of molecules of subcellular structures, as well as proteins and nucleic acids.
Water has a large heat capacity. That is, it absorbs a large amount of heat energy with a minimal increase in its own temperature.
This is achieved due to the fact that a large amount of energy is spent on breaking hydrogen bonds. The high heat capacity of water protects organisms from overheating. And in addition, it creates permanent conditions for the flow of biochemical processes in the body.
Water has a high thermal conductivity, which will ensure an even distribution of heat throughout the body. Due to this, biochemical processes and all processes of vital activity take place in relatively constant conditions.
Water has a relatively high heat of evaporation. The evaporation of water is accompanied by cooling of the body, because a large amount of energy is spent on breaking hydrogen bonds, and this energy is drawn from the environment.
Water is practically not compressed, thus creating turgor pressure, determining the volume and elasticity of cells and tissues. For example, because of this, our skin is elastic, and roundworms and jellyfish have a hydrostatic skeleton.
Water is characterized by high surface tension, which is associated with the formation of hydrogen bonds between water molecules and other compounds.


Use of surface tension by living organisms. A water skater runs through the water (left). Blood moves through the capillary (right)
Due to the force of surface tension of water, capillary blood flow occurs in our body, ascending and descending currents of water in the body of plants. Many small organisms benefit from this surface tension, which allows them to stay on the water or glide on its surface.

The main biologically important properties of water
Thus, we have considered the structure and properties of water.
The value of sweating
Sweating is the release of liquid secretions on the surface of the skin. Together with sweat, substances such as amino acids, Soaps, fatty acids, ammonia, and cholesterol are released – substances that are products of the vital activity of living organisms. Also, heavy metal ions that accidentally enter the body can be released with sweat
The composition of sweat on different parts of the human body is not the same, and depends on different factors: the state of the body, the type of food, the action of other factors (for example, humidity, temperature), as well as physical activity.
There are thermal and psychogenic sweating.
Thermal sweating, depending on the ambient temperature, is one of the mechanisms of thermoregulation, that is, it saves our body from overheating. Thermal sweating develops within a few minutes.
Psychogenic sweating depends on the emotional state.
Psychogenic sweating can develop on different parts of the human body, it can even be the soles of the feet, the pads of the fingers; and develops within a few seconds.
The density of water and the behavior near the freezing point
The maximum water density is at +4oC. It decreases from +4 to 0, meaning that ice is less dense (and therefore lighter) than water.


Ice floating on the water surface (left) and organisms living under the ice (right)
This is of great importance for living organisms that live in water, because reservoirs freeze from above, and many organisms remain viable in them under the ice.
If reservoirs were frozen from the bottom, then all these living organisms would die in the winter.