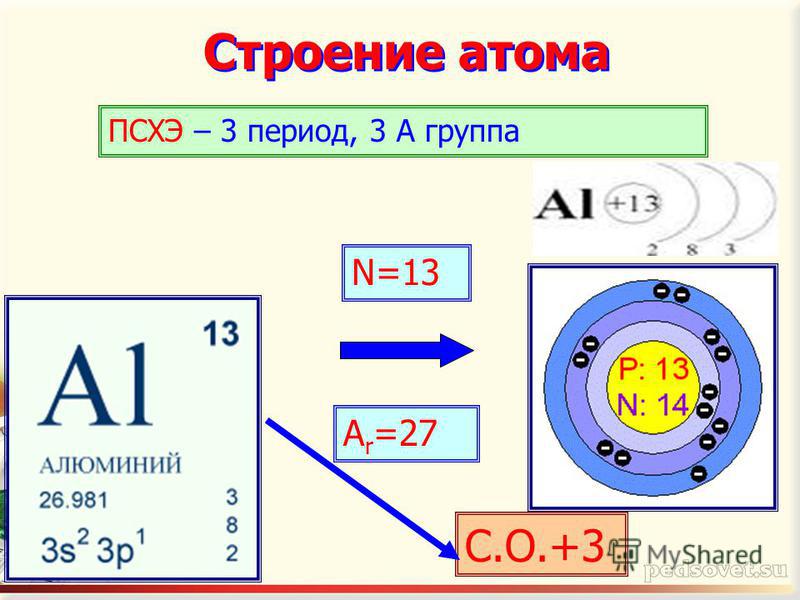
| Организационный момент Good morning, everybody. Today we will study chemistry using our English language. Good luck with your lesson. Let's start our lesson right now. Погружение в тему Учитель показывает изделия из алюминия, просит посмотреть на слайд, задает вопросы и читает стихотворение. What chemical element did D.I. Mendeleev call the metal of the future? What element is it talking about? I am an irreplaceable metal, Very much a pilot's favorite, Lightweight, Electrically conductive, And the character is transitional.
Look at these products, what metal are they made of? Do you know what the hulls of cars, planes and missiles are made of? What are electrical wires made of? Where is aluminum used? 
Ознакомление с темой урока, целями обучения и критериями обучения Today in the lesson you will learn about the physical and chemical properties of aluminum . -Learn how to make chemical equations of reactions -Write the equations of possible reactions. 
Опрос знаний учащихся по предыдущей теме. Let's start repeating the homework. You need to remember and tell briefly about alkaline and alkaline earth metals. Remember their structure and properties. Учитель раздает ученикам стикеры разного цвета и делит класс на 3 группы. 1 group. General characteristics of metals. The position in the periodic table. Obtaining metals . Общая характеристика металлов. Положение в таблице Менделеева. Получение металлов Group 2. Alkali metals and their properties. Щелочные металлы и их свойства 3rd group Alkali - earth metals and their properties Щелочно - земельные металлы и их свойства. По одному ученику из группы выходят к доске для записи уравнений химических реакций One student from the group goes to the blackboard to write down the equations of chemical reactions . Учитель раздает ученикам тестовые задания | Choose the correct answer | |
| Alkaline earth metals are in: 1) I A group; 2) II A group; 3) IV A group 4) VIII A group | |
| Which of these metals is alkaline: 1) Mg; 2) Zn; 3) Ba; 4) K | |
| Degree of oxidation of alkali metals: +1; 2) +2; 3) -2; 4) +3 | |
| Distribution of electrons by energy levels in a magnesium atom: 1) 2,8,2; 2) 2,8,1; 3) 2,8,8,1; 4) 1,8,8,1 | |
| Alkaline earth metals: A. Silver – white. B. Easily cut with a knife. B. Inactive metals. 1) All statements are not true; 2) True A and B; 3) True A and C | |
| When interacting with oxygen, sodium forms: 1) oxide; 2) peroxide; 3) sodium does not react with oxygen. | Давайте проверим, как вы выполнили тест.. Прочтите вопросы на доске. Давайте сверим ваши ответы. Let's check how you completed the test. . Read the questions on the board.Let 's compare your answers |