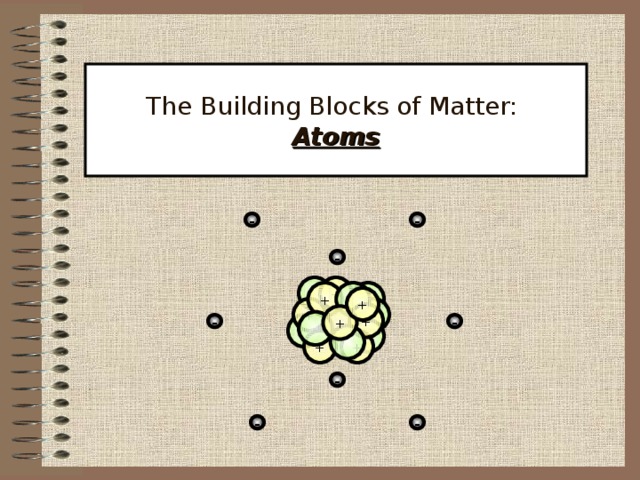
The Building Blocks of Matter: Atoms
-
-
-
+
+
+
+
+
+
-
-
+
+
-
-
-
Matter
- Anything that has mass and takes up space (volume)
- A brick has mass and takes up space A desk has mass and takes up space A pencil has mass and takes up space Air has mass and takes up space
- A brick has mass and takes up space A desk has mass and takes up space A pencil has mass and takes up space Air has mass and takes up space
- A brick has mass and takes up space
- A desk has mass and takes up space
- A pencil has mass and takes up space
- Air has mass and takes up space
All of the above examples are considered matter because they have mass and take up space . Can you think of anything that would not be considered matter?
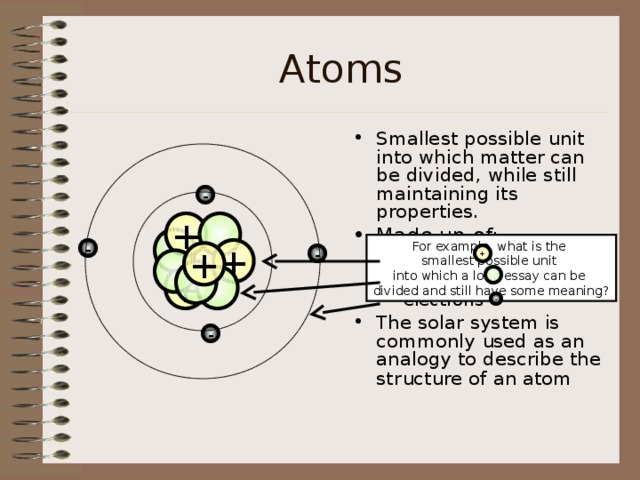
Atoms
- Smallest possible unit into which matter can be divided, while still maintaining its properties.
- Made up of:
- protons neutrons electrons
- protons
- neutrons
- electrons
- The solar system is commonly used as an analogy to describe the structure of an atom
-
+
For example, what is the
smallest possible unit
into which a long essay can be
divided and still have some meaning?
+
-
+
+
-
+
-
-

Atoms are so small that…
- it would take a stack of about 50,000 aluminum atoms to equal the thickness of a sheet of aluminum foil from your kitchen.
- if you could enlarge a penny until it was as wide as the US, each of its atoms would be only about 3 cm in diameter – about the size of a ping-pong ball
- a human hair is about 1 million carbon atoms wide.
- a typical human cell contains roughly 1 trillion atoms.
- a speck of dust might contain 3x10 12 (3 trillion) atoms.
- it would take you around 500 years to count the number of atoms in a grain of salt.
www.deckersfoods.com
C-C-C-C-C-… + 999,995 more
1 trillion atoms
.
Is made of approximately 3 trillion atoms
Just one of these grains

Let’s Experiment
In order to try to gain an idea of how small an atom really is, you will complete the following activity.
- Cut a strip of 11 in. paper in half. Discard one half. Cut the remaining piece in half. Continue cutting and discarding the strips as many times as you can. Make all cuts parallel to the first one. When the width gets longer than the length, you may cut off the excess, but that does not count as a cut.
- Cut a strip of 11 in. paper in half.
- Discard one half.
- Cut the remaining piece in half.
- Continue cutting and discarding the strips as many times as you can.
- Make all cuts parallel to the first one. When the width gets longer than the length, you may cut off the excess, but that does not count as a cut.

Results
- How many cuts were you able to make?
- Do you think you could keep cutting the paper forever? Why or why not?
You would have to cut the paper in half around thirty-one (31) times to get to the size of any atom.
http://www.miamisci.org/af/sln/phantom/papercutting.html
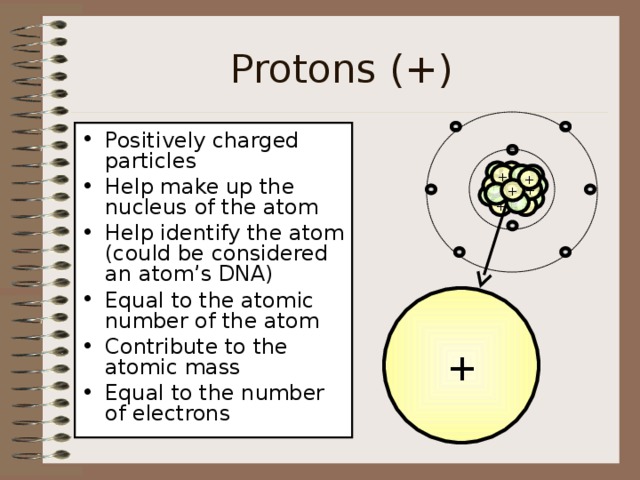
Protons (+)
- Positively charged particles
- Help make up the nucleus of the atom
- Help identify the atom (could be considered an atom’s DNA)
- Equal to the atomic number of the atom
- Contribute to the atomic mass
- Equal to the number of electrons
-
-
-
+
+
+
+
+
+
-
-
+
+
-
-
-
+
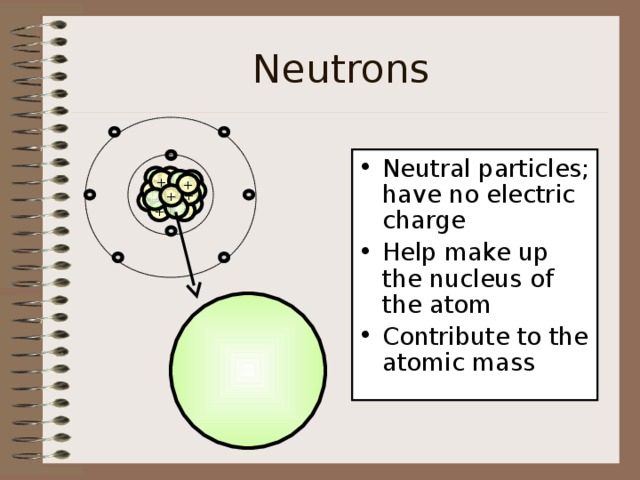
Neutrons
-
-
- Neutral particles; have no electric charge
- Help make up the nucleus of the atom
- Contribute to the atomic mass
-
+
+
+
+
+
+
-
-
+
+
-
-
-
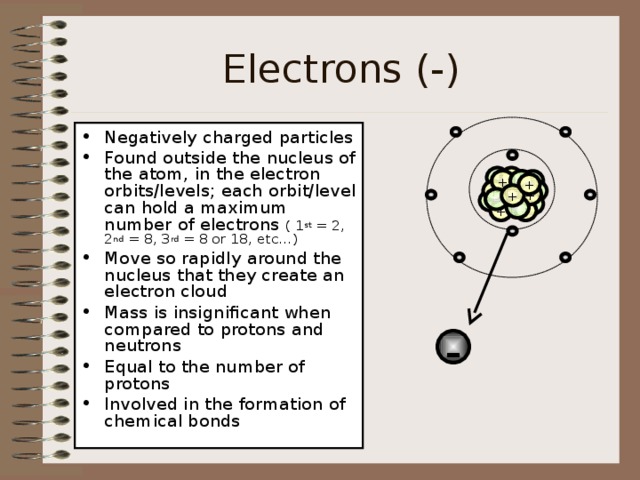
Electrons (-)
- Negatively charged particles
- Found outside the nucleus of the atom, in the electron orbits/levels; each orbit/level can hold a maximum number of electrons ( 1 st = 2, 2 nd = 8, 3 rd = 8 or 18, etc…)
- Move so rapidly around the nucleus that they create an electron cloud
- Mass is insignificant when compared to protons and neutrons
- Equal to the number of protons
- Involved in the formation of chemical bonds
-
-
-
+
+
+
+
+
+
-
-
+
+
-
-
-
-
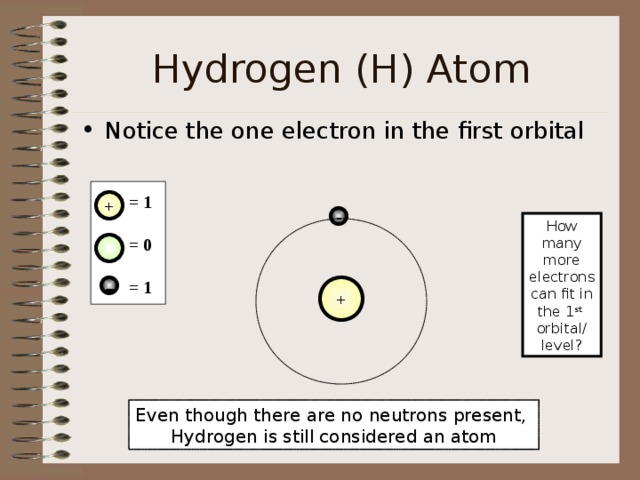
Hydrogen (H) Atom
- Notice the one electron in the first orbital
= 1
= 0
= 1
+
-
How many
more electrons
can fit in the 1 st
orbital/ level?
+
-
Even though there are no neutrons present,
Hydrogen is still considered an atom
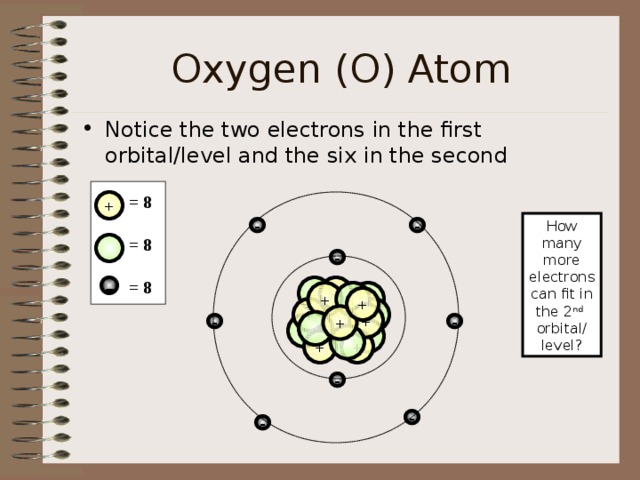
Oxygen (O) Atom
- Notice the two electrons in the first orbital/level and the six in the second
= 8
= 8
= 8
+
How many
more electrons
can fit in the 2 nd
orbital/ level?
-
-
-
-
+
+
+
+
+
+
-
-
+
+
-
-
-
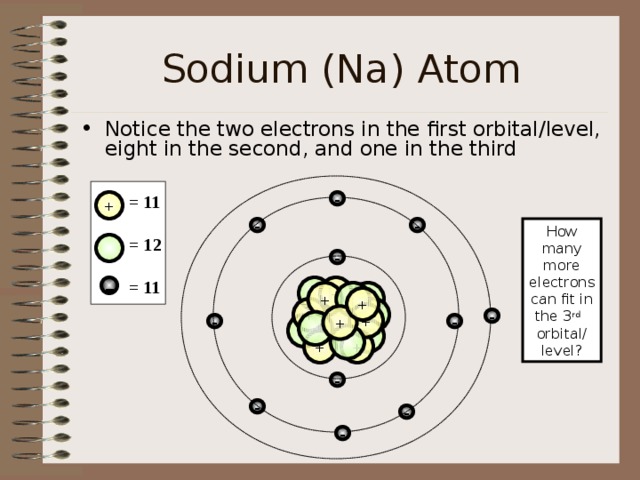
Sodium (Na) Atom
- Notice the two electrons in the first orbital/level, eight in the second, and one in the third
= 11
= 12
= 11
+
-
-
-
How many
more electrons
can fit in the 3 rd
orbital/ level?
-
+
-
+
+
+
+
+
-
-
-
+
+
-
-
-
-
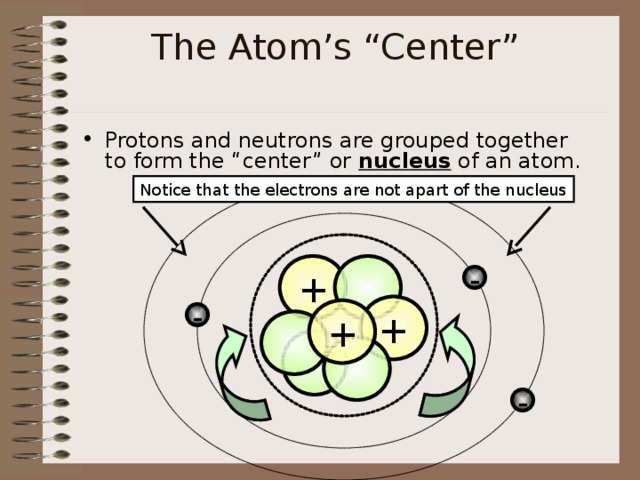
The Atom’s “Center”
- Protons and neutrons are grouped together to form the “center” or nucleus of an atom.
Notice that the electrons are not apart of the nucleus
+
-
+
+
-
-
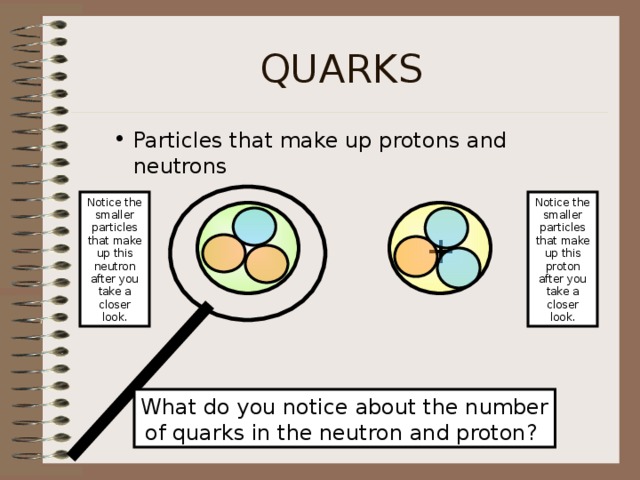
QUARKS
- Particles that make up protons and neutrons
- Particles that make up protons and neutrons
Notice the smaller particles that make up this neutron after you take a closer look.
Notice the smaller particles that make up this proton after you take a closer look.
+
What do you notice about the number
of quarks in the neutron and proton?
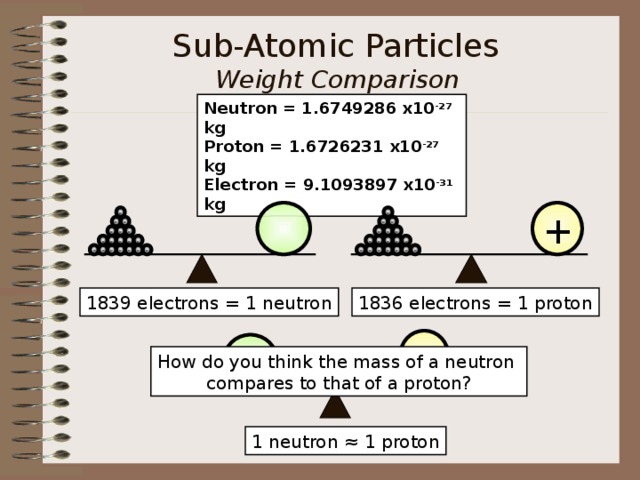
Sub-Atomic Particles Weight Comparison (protons, neutrons, electrons)
Neutron = 1.6749286 x10 -27 kg Proton = 1.6726231 x10 -27 kg Electron = 9.1093897 x10 -31 kg
+
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
1836 electrons = 1 proton
1839 electrons = 1 neutron
+
How do you think the mass of a neutron
compares to that of a proton?
1 neutron ≈ 1 proton
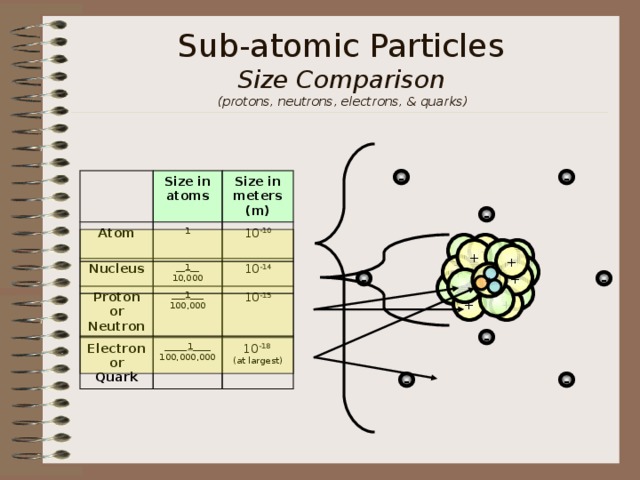
Sub-atomic Particles Size Comparison (protons, neutrons, electrons, & quarks)
-
-
Size in atoms
Atom
Nucleus
1
Size in meters (m)
__ 1 __
10,000
10 -10
Proton or Neutron
Electron or Quark
___ 1 ___
100,000
10 -14
_____ 1 ____
100,000,000
10 -15
10 -18
(at largest)
-
+
+
+
+
+
+
-
-
+
+
-
-
-
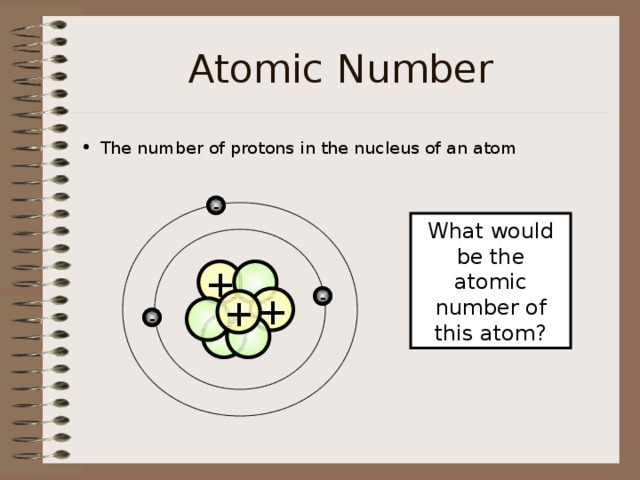
Atomic Number
- The number of protons in the nucleus of an atom
-
What would be the atomic number of this atom?
+
-
+
+
-
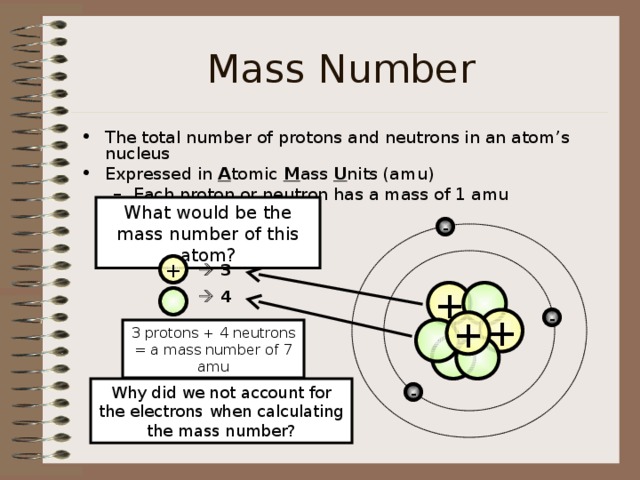
Mass Number
- The total number of protons and neutrons in an atom’s nucleus
- Expressed in A tomic M ass U nits (amu)
- Each proton or neutron has a mass of 1 amu
- Each proton or neutron has a mass of 1 amu
What would be the mass number of this atom?
-
+
3
+
4
-
+
+
3 protons + 4 neutrons = a mass number of 7 amu
Why did we not account for the electrons when calculating the mass number?
-
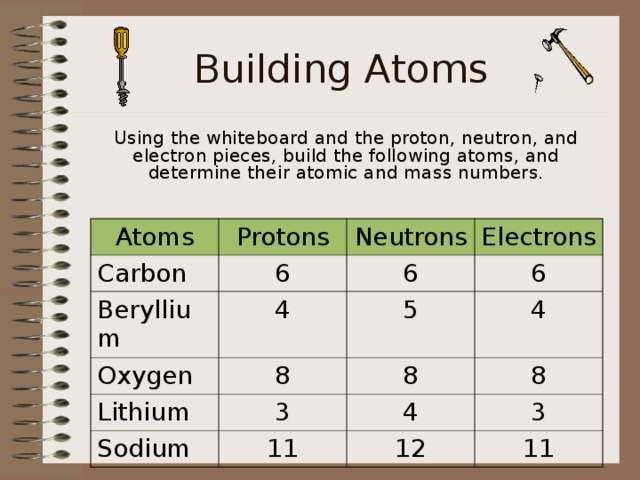
Building Atoms
Using the whiteboard and the proton, neutron, and electron pieces, build the following atoms, and determine their atomic and mass numbers.
Atoms
Protons
Carbon
Beryllium
Neutrons
6
Oxygen
Electrons
4
6
Lithium
5
8
6
8
Sodium
4
3
8
4
11
3
12
11
Atom Builder
- Using the interactive website link below, practice building atoms.
- http://www.pbs.org/wgbh/aso/tryit/atom/
- Using the classzone.com link below, click on the “Build an Atom” simulation and practice building atoms.
http:// www.classzone.com/books/ml_sci_physical/page_build.cfm?id=resour_ch1&u=2##

FORCES IN THE ATOM
- Gravitational Force
- Electromagnetic Force
- Strong Force
- Weak Force
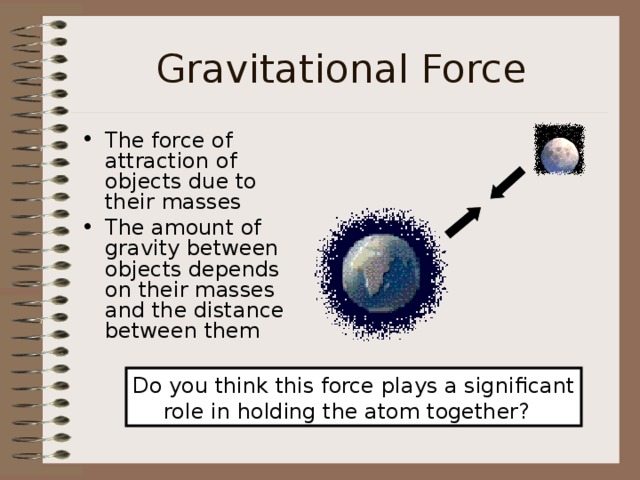
Gravitational Force
- The force of attraction of objects due to their masses
- The amount of gravity between objects depends on their masses and the distance between them
Do you think this force plays a significant
role in holding the atom together?
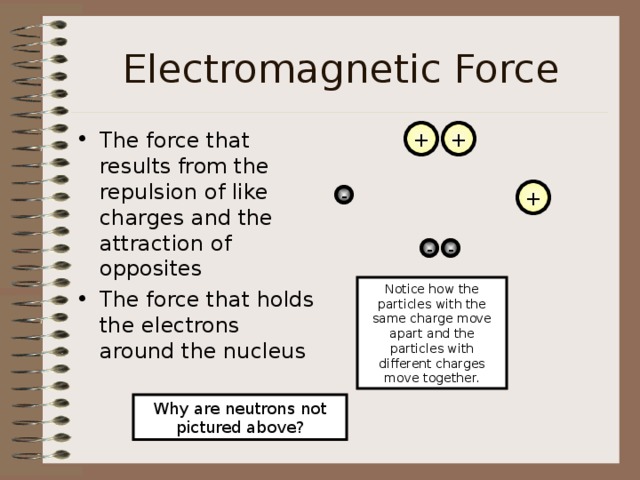
Electromagnetic Force
- The force that results from the repulsion of like charges and the attraction of opposites
- The force that holds the electrons around the nucleus
+
+
+
-
-
-
Notice how the particles with the same charge move apart and the particles with different charges move together.
Why are neutrons not pictured above?
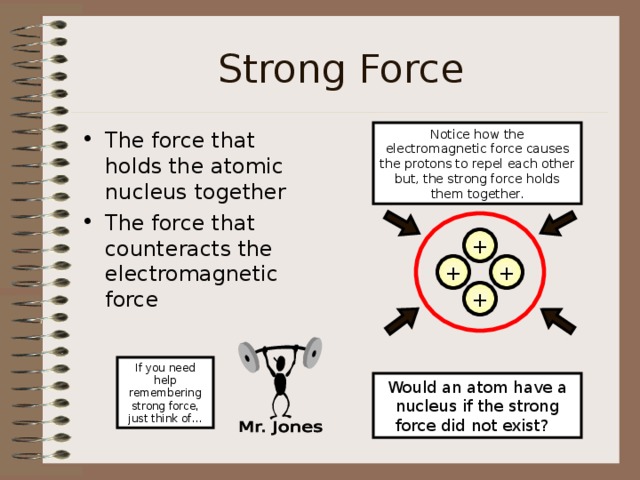
Strong Force
Notice how the electromagnetic force causes the protons to repel each other but, the strong force holds them together.
- The force that holds the atomic nucleus together
- The force that counteracts the electromagnetic force
+
+
+
+
If you need help remembering strong force, just think of…
Would an atom have a nucleus if the strong force did not exist?
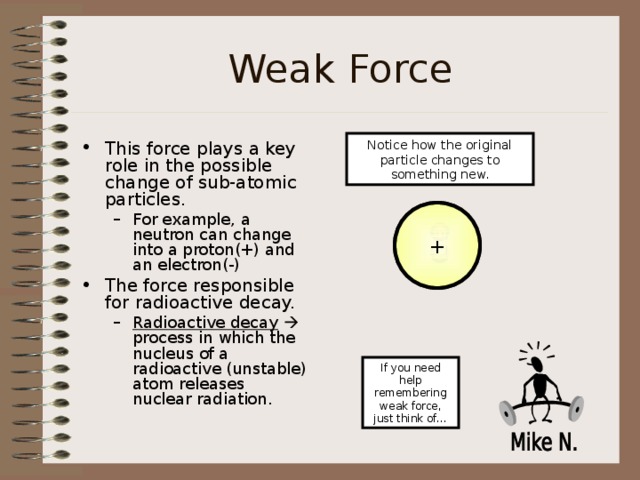
Weak Force
Notice how the original particle changes to something new.
- This force plays a key role in the possible change of sub-atomic particles.
- For example, a neutron can change into a proton(+) and an electron(-)
- For example, a neutron can change into a proton(+) and an electron(-)
- The force responsible for radioactive decay.
- Radioactive decay process in which the nucleus of a radioactive (unstable) atom releases nuclear radiation.
- Radioactive decay process in which the nucleus of a radioactive (unstable) atom releases nuclear radiation.
+
n
-
If you need help remembering weak force, just think of…
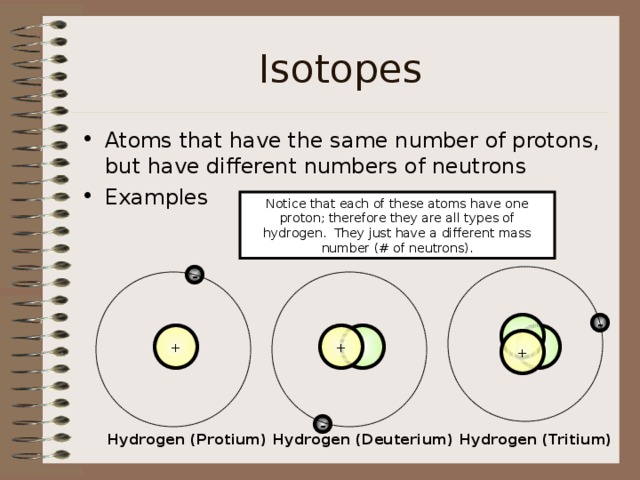
Isotopes
- Atoms that have the same number of protons, but have different numbers of neutrons
- Examples
Notice that each of these atoms have one proton; therefore they are all types of hydrogen. They just have a different mass number (# of neutrons).
-
-
+
+
+
-
Hydrogen (Protium)
Hydrogen (Deuterium)
Hydrogen (Tritium)
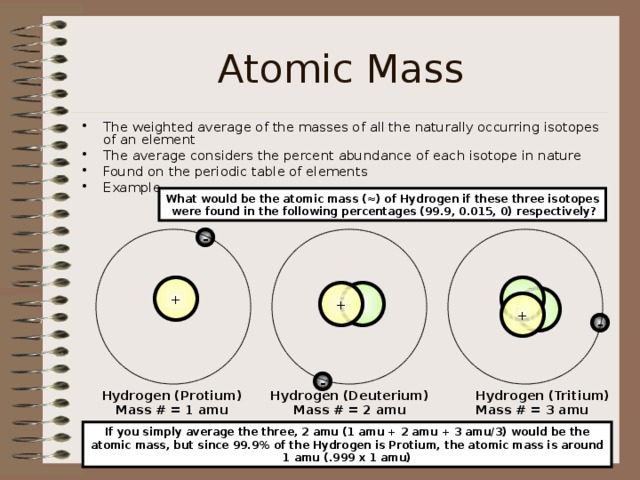
Atomic Mass
- The weighted average of the masses of all the naturally occurring isotopes of an element
- The average considers the percent abundance of each isotope in nature
- Found on the periodic table of elements
- Example
What would be the atomic mass (≈) of Hydrogen if these three isotopes
were found in the following percentages (99.9, 0.015, 0) respectively?
-
+
+
+
-
-
Hydrogen (Protium)
Mass # = 1 amu
Hydrogen (Deuterium)
Mass # = 2 amu
Hydrogen (Tritium)
Mass # = 3 amu
If you simply average the three, 2 amu (1 amu + 2 amu + 3 amu/3) would be the atomic mass, but since 99.9% of the Hydrogen is Protium, the atomic mass is around 1 amu (.999 x 1 amu)
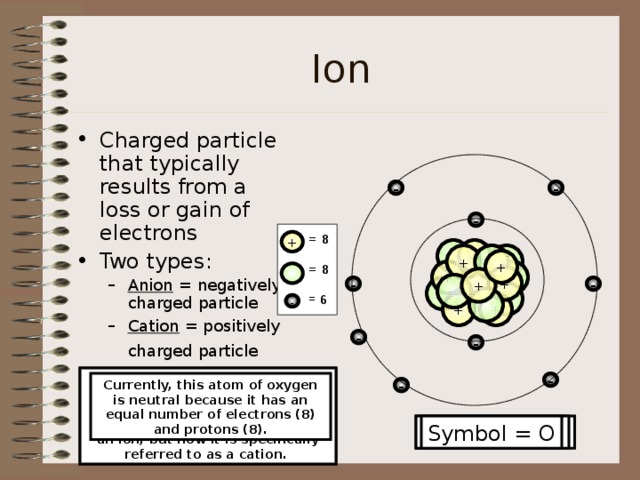
Ion
- Charged particle that typically results from a loss or gain of electrons
- Two types:
- Anion = negatively charged particle Cation = positively charged particle
- Anion = negatively charged particle
- Cation = positively charged particle
-
-
-
= 8
= 8
= 8
+
+
+
+
+
+
+
-
-
6
9
+
+
-
-
-
Now that three electrons were lost, the number of electrons (6) and protons (8) is still unbalanced; therefore, it is still an ion, but now it is specifically referred to as a cation.
Now that this atom of oxygen just gained an electron, it is no longer neutral or an atom. It is now considered an ion (anion). This ion has more electrons (9) than protons (8).
-
Currently, this atom of oxygen is neutral because it has an equal number of electrons (8) and protons (8).
-
Symbol = O 2+
Symbol = O 1-
Symbol = O
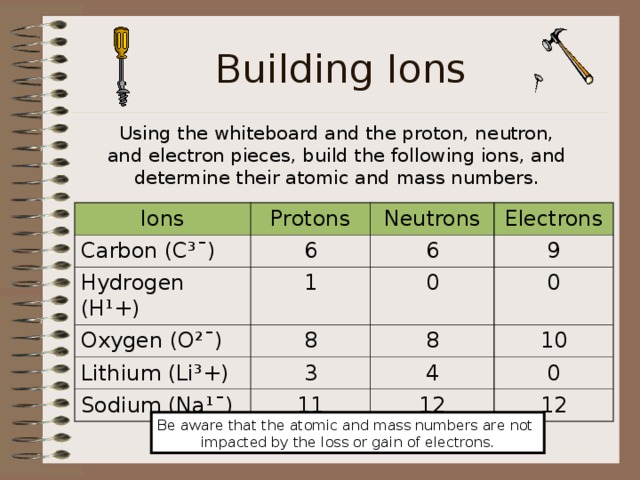
Building Ions
Using the whiteboard and the proton, neutron, and electron pieces, build the following ions, and determine their atomic and mass numbers.
Ions
Protons
Carbon (C³ ¯)
Hydrogen (H ¹+)
Neutrons
6
Oxygen (O ²¯)
Electrons
1
6
Lithium (Li³+)
9
8
0
Sodium (Na ¹¯)
0
8
3
4
10
11
0
12
12
Be aware that the atomic and mass numbers are not
impacted by the loss or gain of electrons.